The Fasting Mimicking Diet and its Relationship to Alzheimer’s Disease
by Brooklin White MS, RDN, LDNNutrition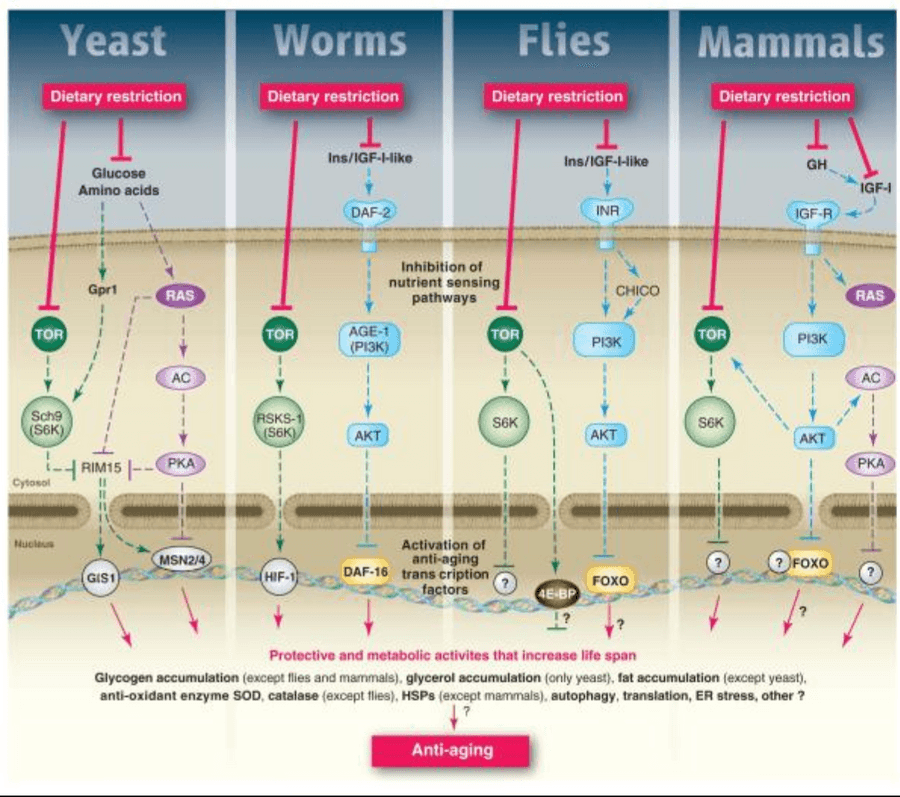
The Fasting Mimicking Diet
The fasting mimicking diet (FMD) is a periodic macro and micronutrient defined 5-day regimen that induces fasting-like benefits. The FMD consists of a limited amount of plant-based foods to ensure nourishment while still obtaining the benefits of a fast. The FMD is a form of calorie restriction that slows down the ongoing “growth” pathways in our cells to make time for repair and regeneration of healthy stem cells.
The FMD was created by Dr. Valter Longo, who is the Director of the Longevity Institute at the University of Southern California. Dr. Longo has dedicated his entire career to understanding the mechanisms behind aging, calorie restriction, nutrition, healthspan and longevity. Various anti-aging effects have been seen with calorie restriction including increased insulin sensitivity and mitochondrial function and decreased oxidative stress and inflammation (1). Data from epidemiological studies also indicate that communities who naturally have lower calorie diets have a reduced risk for developing Alzheimer’s disease, as compared to those with high calorie diets (2). Dr. Longo’s work is rooted in the science of aging and the molecular biology behind it.
The Molecular Biology Behind the FMD: The Growth and Repair Pathways
The mechanisms of aging have been established by numerous NIH funded preclinical (animal) and clinical (human) trials. For example, certain molecular pathways within our cells are told to either grow or repair, depending on the caloric state (3). The growth pathways (AMPK, PI3K, RAS, S6K, PKA) are turned on by the macronutrients we consume such as carbohydrates and protein. When these growth pathways are turned on, they inhibit transcription factors (FOXO) which initiate the repair of damaged cells and the rejuvenation of new stem cells. When we are in a fasting state, such as with the FMD, our growth pathways are downregulated and our repair pathways (beginning with transcription factors like FOXO) are upregulated to active key molecules to clear out DNA mutations and harmful reactive oxygen species (ROS) such as superoxide and hydrogen peroxide. It’s important to note that if one pathway is turned on, then the other is turned off. While humans are definitely a more advanced species, yeast, worms, flies, mice, primates and humans have consistently shown the same growth and repair pathways (3). Although the players are not always identical, the underlying molecular strategy is the same (SCH9 in yeast is analogous to S6K in humans, for example). See the image above for more details.
The problem in western culture today is that our growth pathways are always turned on. We are never without calories and we are often told that the more protein we eat, the better. Unfortunately, this leaves our repair pathways with little time to work. The growth pathways are turned on specifically through growth factors such as Insulin-like growth factor (IGF-1) which comes from a high consumption of proteins and sugars. Mitochondrial superoxide and hydrogen peroxide are byproducts of these growth pathways and major contributors of DNA mutations which lead to disease states and accelerated aging (4). The FMD thus turns off these growth pathways and promotes the repair pathways to release molecules such as superoxide dismutase, to clear out harmful molecules, destroy damaged cells and allow for the regeneration of new cells.
It’s important to note that IGF-1 is not a bad molecule as it is used by our cells for proper growth and development. It’s the over accumulation of IGF-1 attributed to our high caloric diets that cause metabolic issues.
How does the FMD relate to Alzheimer’s Disease?
Although aging encourages a natural wear and tear on our cells, increasing evidence suggests that taking care of our bodies through certain lifestyle behaviors can make dramatic impacts on our health long term (3)(5). Since the FMD encourages the clearing of damaged cells and the regeneration of new stem cells, research is indicating that periodic cycles of the FMD could serve as protection against neurodegenerative disorders (6). Dietary restriction in general has been shown to slow down the onset of neurodegenerative diseases by increasing the resistance of neurons to degeneration (7).
With dietary restriction, the peripheral organs (those outside of the brain) see a decrease in plasma levels of glucose, insulin, homocysteine and cholesterol, which ultimately show beneficial effects on the brain’s vascular cells, neurons and glia (glia serve as supporting cells to neurons). The brain then experiences mild metabolic stress which leads to a gene expression response such as an increase in neurotrophic factors such as BDNF*, protein chaperones*, anti-apoptotic proteins* and synaptic proteins*. The combination of both the peripheral and brain effects of dietary restriction lead to neuron survival, synaptic strength and neurogenesis (7).
Preclinical Trials
- Brandhorst et al., found that 4 days of a FMD twice a month promoted hippocampal neurogenesis, lowered IGF-1, and improved cognition in older mice (6).
- Brandhorst et al., also demonstrated that after 72 hours on the FMD there was a reduction in circulating hippocampal IGF-1 but increased IGF-1 receptor in the dentate gyrus region of the hippocampus. The dentate gyrus is the location where neurogenesis occurs. This means that when the mice were refed (after the FMD) the incoming IGF-1 from their diet was immediately used for rebuilding synapses and new neural stem cells. This study also showed that the PKA pathway (one of the growth pathways) was reduced (6).
- Parrella et al. showed that a low-protein diet can slow the progression of Alzheimer's Disease in mice by lowering circulating levels of IGF-1 (8).
- Sanz et al., showed that the oxidative damage to nuclear DNA in old mice brains was fully reversed by caloric restriction to the level of old controls. Brain oxidative modifications/damage increases with age and decreases with CR (9)
- Both mice and primate studies have shown that calorie restriction reduces amyloid-beta accumulation (10)(11)(12)
- The SIRT1-mediated deacetylation of FOXO (transcription factor activated in the repair pathway) in response to calorie restriction has been shown to decrease amyloid-beta production. Since FOXO centrally regulates autophagy (the body’s way of clearing out damaged cells) it is hypothesized that this mechanism resulting from calorie restriction could help in the prevention and treatment of Alzheimer’s Disease (13)
Clinical Trials
- Recent clinical trials have shown that the FMD is safe and well tolerated in healthy subjects, even those over 65. These trials have shown reduced risks for various aging diseases including Alzheimer’s Disease, arterial hypertension, pre-diabetes, accumulation of adipose tissue and high circulating levels of IGF-1 and CRP (14).
- Brandhorst et al., found that three monthly cycles of the FMD reduced multiple risk factors of aging including reduced blood glucose levels, reduced circulating IGF-1 levels, reduced C-Reactive Protein levels (marker of inflammation) and an increase in serum ketone bodies (4).
- When looking at research it is crucial to look at hypotheses from every angle. A community based in Ecuador known as the Laron’s are known to have a genetic deficiency in growth hormone receptors – meaning their cells do not uptake IGF-1 like other individuals. Although this growth hormone receptor deficiency stunts their growth, it also prevents them from ever getting cancer, type 2 diabetes, and Alzheimer’s disease. A recent study was done on the Laron’s at USC to measure brain function and connectivity between the growth-hormone receptor deficiency (GHRD) Laron’s and their unaffected relatives. This study found that the GHRD individuals showed trends toward larger dentate gyrus and CA1 regions of the hippocampus, which are responsible for neurogenesis. The GHRD group showed enhanced cognitive performance and greater task related activation in frontal, parietal and hippocampal regions as compared with controls (15).
- A trial by Dr. Longo composed of 120 older adults with mild MCI or early stage AD is currently being conducted to determine if the FMD has the same neuroprotective and regenerative effects as shown in the preclinical trials. The trial is predicted to end in December of 2021 (16).
Research on the FMD is not only looking at the benefits of cognition and Alzheimer’s Disease, but it is also starting to show exciting results in clinical trials when it comes to fighting cancer, diabetes, obesity, various autoimmune diseases including multiple sclerosis, and aging in general. The FMD’s underlying mechanism of action targets the molecular pathways responsible for aging and thus aids in the prevention and reversal of diseases associated with natural decline. The FMD is truly a form of communication to your cells that triggers the repair pathways to clear damage and allow for the regeneration of new stem cells. One small way you can trigger your repair pathways right away is to follow the KetoFlex 12/3 diet as part of the ReCODE protocol. The 12/3 refers to eating within a 12 hour window and stopping food consumption 3 hours before you go to bed. This allows your cells to shift gears and maximize their time in the repair state.
Although the clinical trials in Alzheimer’s Disease and FMD are ongoing, the results from the preclinical trials composed of animals with the same growth and repair pathways, are inspiring. If you are interested in how you can implement the FMD while following the Bredesen Protocol, reach out to your registered dietitian at the Amos Institute today.
*Synaptic proteins: regulate neurotransmitter release and participate in the early development of neurons
*Antiapoptotic proteins: preserve mitochondrial integrity
*BDNF: protects neuronal cells and pancreatic B-cells
*Protein chaperones: aids in the proper folding of proteins and the prevention of protein aggregation
References
Image source: Fontana, Partridge and Longo, 2010
- Fontana, L., Nehme, J., & Demaria, M. (2018). Caloric restriction and cellular senescence.Mechanisms of Ageing and Development,176, 19–23.https://doi.org/10.1016/j.mad.2018.10.005
- Luchsinger, J. A., Tang, M.-X., Shea, S., & Mayeux, R. (2002). Caloric Intake and the Risk of Alzheimer Disease.Archives of Neurology,59(8), 1258–1263.https://doi.org/10.1001/archneur.59.8.1258
- Fontana, L., Partridge, L., & Longo, V. D. (2010). Dietary Restriction, Growth Factors and Aging: From yeast to humans.Science (New York, N.Y.),328(5976), 321–326.https://doi.org/10.1126/science.1172539
- Szeto, H.H. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J 8, E277–E283 (2006).https://doi.org/10.1007/BF02854898
- Earls, J. C., Rappaport, N., Heath, L., Wilmanski, T., Magis, A. T., Schork, N. J., Omenn, G. S., Lovejoy, J., Hood, L., & Price, N. D. (2019). Multi-Omic Biological Age Estimation and Its Correlation With Wellness and Disease Phenotypes: A Longitudinal Study of 3,558 Individuals.The Journals of Gerontology: Series A,74(Supplement_1), S52–S60.https://doi.org/10.1093/gerona/glz220
- Brandhorst, S., Choi, I. Y., Wei, M., Cheng, C. W., Sedrakyan, S., Navarrete, G., Dubeau, L., Yap, L. P., Park, R., Vinciguerra, M., Di Biase, S., Mirzaei, H., Mirisola, M. G., Childress, P., Ji, L., Groshen, S., Penna, F., Odetti, P., Perin, L., … Longo, V. D. (2015). A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan.Cell Metabolism,22(1), 86–99.https://doi.org/10.1016/j.cmet.2015.05.012
- Mattson, M. P. (2003). Gene–Diet Interactions in Brain Aging and Neurodegenerative Disorders.Annals of Internal Medicine,139(5Part2), 441.https://doi.org/10.7326/0003-4819-139-5_Part_2-200309021-00012
- Parrella, E., Maxim, T., Maialetti, F., Zhang, L., Wan, J., Wei, M., Cohen, P., Fontana, L., & Longo, V. D. (2013). Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model.Aging Cell,12(2), 257–268.https://doi.org/10.1111/acel.12049
- Sanz, A., Caro, P., Ibañez, J., Gómez, J., Gredilla, R., & Barja, G. (2005). Dietary Restriction at Old Age Lowers Mitochondrial Oxygen Radical Production and Leak at Complex I and Oxidative DNA Damage in Rat Brain.Journal of Bioenergetics and Biomembranes,37(2), 83–90.https://doi.org/10.1007/s10863-005-4131-0
- Qin, W., Chachich, M., Lane, M., Roth, G., Bryant, M., de Cabo, R., Ottinger, M. A., Mattison, J., Ingram, D., Gandy, S., & Pasinetti, G. M. (2006). Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus).Journal of Alzheimer’s Disease,10(4), 417–422.https://doi.org/10.3233/JAD-2006-10411
- Wang, J., Ho, L., Qin, W., Rocher, A. B., Seror, I., Humala, N., Maniar, K., Dolios, G., Wang, R., Hof, P. R., & Pasinetti, G. M. (2005). Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer’s disease.The FASEB Journal,19(6), 1–18.https://doi.org/10.1096/fj.04-3182fje
- Patel, N. V., Gordon, M. N., Connor, K. E., Good, R. A., Engelman, R. W., Mason, J., Morgan, D. G., Morgan, T. E., & Finch, C. E. (2005). Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models.Neurobiology of Aging,26(7), 995–1000.https://doi.org/10.1016/j.neurobiolaging.2004.09.014
- Qin, W., Zhao, W., Ho, L., Wang, J., Walsh, K., Gandy, S., & Pasinetti, G. M. (2008). Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration.Annals of the New York Academy of Sciences,1147, 335–347.https://doi.org/10.1196/annals.1427.024
- Wei, M., Brandhorst, S., Shelehchi, M., Mirzaei, H., Cheng, C. W., Budniak, J., Groshen, S., Mack, W. J., Guen, E., Di Biase, S., Cohen, P., Morgan, T. E., Dorff, T., Hong, K., Michalsen, A., Laviano, A., & Longo, V. D. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease.Science Translational Medicine,9(377).https://doi.org/10.1126/scitranslmed.aai8700
- Nashiro, K., Guevara-Aguirre, J., Braskie, M. N., Hafzalla, G. W., Velasco, R., Balasubramanian, P., Wei, M., Thompson, P. M., Mather, M., Nelson, M. D., Guevara, A., Teran, E., & Longo, V. D. (2017). Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans.Journal of Neuroscience,37(7), 1696–1707.https://doi.org/10.1523/JNEUROSCI.1929-16.2016
- Randomized phase I / II study of a hypoproteic diet in patients with cognitive impairment [List of Clinical Trials. L-Nutra]